- Igiene e sicurezza
- 1 likes
- 12074 views
Sterilization is the final result of a process designed to ensure the annihilation of living microorganisms on a surface. An instrument, an object will be considered sterile when the chances of finding a microorganism are less than one in a million.
The sterilization procedure
The most consolidated sterilization procedure in the literature, which in any case may differ according to the individual operating situations, consists of the following 18 points summarized in brief:
1. COLLECTION / TRANSPORT
By collection and / or transport we mean the removal of the instruments used and their movement from the place of operation to that dedicated to the sterilization process. This too must take place in safety for the operator who must be equipped with adequate individual protections (gloves, gown, mask, goggles, etc ...). In the most risky cases, the collection of the medical devices used must take place in a rigid container without welding with 2 side handles and removable grid (so-called safety containers).
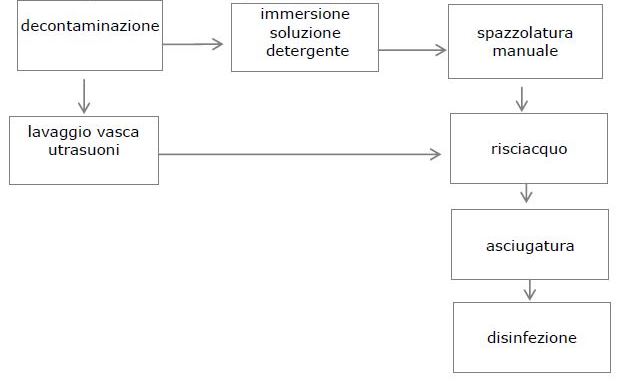
2. DECONTAMINATION
After use, the instruments must not be handled to avoid the risk of injury that opens the way for the entry of pathogenic germs (eg HIV, hepatitis B and C virus), but must be immediately immersed in a decontaminating solution containing an agent. chemical disinfectant in order to decontaminate them.
Read about decontamination and washing of tools
3. WASHING
After the first decontamination phase, you can proceed, always wearing gloves and goggles, to the washing procedure which aims to remove the residues of organic and inorganic substances and consequently, also the microorganisms.
The procedure for manual washing consists of 3 phases:
IMMERSION
MANUAL BRUSHING
FLUSHING
DRYING
This is one of the most important "preparatory" phases of the sterilization process.
To learn more about the topic, read here
4. ULTRASONIC TANK WASH
Washing in the ultrasonic tank is a support treatment to the manual or automatic one but it is not a substitute. The instruments must be completely immersed in the solution, opened and / or disassembled. The concentration of the solution, the water temperature (approx. 40 ° C), the frequency of the ultrasounds and the contact time must be observed. The DMs subjected to washing in an ultrasonic tank must be subsequently rinsed to remove the residues previously removed.
The cleaning solution must be replaced every day and in any case every time it is visibly dirty, cloudy. It is not suitable for elastic instruments, oral mirrors, handpieces and turbines.
Manual or ultrasonic cleaning uses the chemical or enzymatic and, at the same time, non-corrosive action of a detergent that must be used strictly at the concentrations and contact times recommended by the manufacturer.
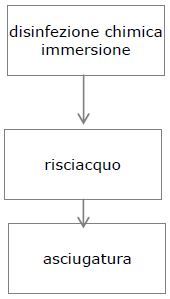
5. DISINFECTION
Disinfection is the next step after the washing step, which is not necessary in the case of using an instrument washer. Disinfection can be chemical or thermal.
There are 3 steps in chemical disinfection:
IMMERSION
FLUSHING
DRYING
Read more about instrument disinfection
In thermal disinfection, a thermostatic bath (thermodisinfector) is used. Thermal disinfection is preferable to chemical disinfection as it is more easily controlled and there is no presence of any chemical residues.
6. AUTOMATIC WASHING: INSTRUMENT WASHING
The automatic washing takes place by means of the INSTRUMENT WASHER (IRON WASHER) which performs washing, disinfection, rinsing and subsequent drying of the DM with standardized programs, this method reduces the possibility of accidents at work.
The instrument washer is indicated in dental surgeries for washing and disinfecting a wide range of materials used, including those with cavities using special accessories. The instrument washer performs both cleaning, disinfection, rinsing and subsequent mechanical drying.
7. CONTROL AND MAINTENANCE OF MEDICAL DEVICES
The devices (tools) must be subjected to visual inspection in order to verify cleanliness, integrity, absence of rust and corrosion. Inspection, re-assembly of the devices that had been dismantled, maintenance and lubrication
8. PACKAGING: STERILE BARRIER SYSTEM (SBS)
Packaging is a preliminary action to sterilization and consists of inserting the devices into sterilization pouches (sterile barrier system - SBS) which have the aim of allowing sterilization, providing physical protection, maintaining sterility up to use. Read how to proceed with the packaging of the instruments in the correct way.
9. NOT PACKAGED MEDICAL DEVICES
Non-autoclaved medical devices can be used. C